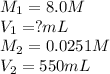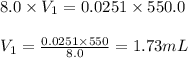A chemist must prepare 550 mL of hydrochloric acid solution with a pH of 1.60 at 25°C .
He wi...

A chemist must prepare 550 mL of hydrochloric acid solution with a pH of 1.60 at 25°C .
He will do this in three steps:
-Fill a 550.0 mL volumetric flask about halfway with distilled water.
-Measure out a small volume of concentrated (8.0M) stock hydrochloric acid solution and add it to the flask.
-Fill the flask to the mark with distilled water.
Calculate the volume of concentrated hydrochloric acid that the chemist must measure out in the second step. Round your answer to significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
Questions in other subjects:

World Languages, 09.04.2021 02:50

Engineering, 09.04.2021 02:50





Mathematics, 09.04.2021 02:50


Mathematics, 09.04.2021 02:50

![pH=-\log[H^+]](/tpl/images/0570/3526/cf945.png)
![1.60=-\log [H^+]](/tpl/images/0570/3526/bdcbd.png)
![[H^+]=10^{-1.60}=0.0251M](/tpl/images/0570/3526/4bcd7.png)
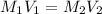
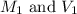 are the molarity and volume of the concentrated HCl solution
are the molarity and volume of the concentrated HCl solution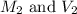 are the molarity and volume of diluted HCl solution
are the molarity and volume of diluted HCl solution