Chemistry, 30.03.2020 04:55 ianball025
A sample of flammable liquid is placed into an enclosed cylinder which is then fitted with a movable piston. Initially the cylinder contains a volume of 1.60 L. The sample is ignited producing gas and releasing 401.5 J of energy. To what volume will the cylinder expand to if it must expand against a pressure of 729.8 mmHg. Assume all the energy released is converted to work used to push the piston?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3
You know the right answer?
A sample of flammable liquid is placed into an enclosed cylinder which is then fitted with a movable...
Questions in other subjects:

History, 21.09.2019 09:00

History, 21.09.2019 09:00

Mathematics, 21.09.2019 09:00




History, 21.09.2019 09:00

Mathematics, 21.09.2019 09:00

Health, 21.09.2019 09:00

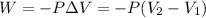
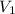 = initial volume = 1.60 L
= initial volume = 1.60 L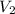 = final volume = ?
= final volume = ?