
Chemistry, 30.03.2020 04:33 smelcher3900
According to the equation below, how many moles of Ca(OH)2 are required to react with 1.36 mol H3PO4 to produce Ca3(PO4)2? 3Ca(OH)2+2H3PO4⟶Ca3(PO4)2+6H2O

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
According to the equation below, how many moles of Ca(OH)2 are required to react with 1.36 mol H3PO4...
Questions in other subjects:

Mathematics, 13.03.2020 03:48

Mathematics, 13.03.2020 03:48

Mathematics, 13.03.2020 03:48

Chemistry, 13.03.2020 03:48




Mathematics, 13.03.2020 03:49


Mathematics, 13.03.2020 03:49

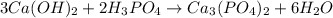
 of calcium hydroxide
of calcium hydroxide

