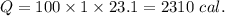Chemistry, 29.03.2020 22:37 Candieboo6939
In lab, a student burns 0.550 grams of Ritz crackers. The burning causes 100.0 grams of water to rise from 24.0°C go 47.1 °C.
Calculate the number of calories absorbed by the WATER as the Ritz crackers burn. (Use the specific heat of water in terms of calories: 1.0 cal/ g °C) ** Remember you are calculating the heat gained by the water, NOT the heat lost by the Ritz cracker - use the correct mass!!
12.7 cal
53.2 cal
2310 cal
9.67 x 105 cal

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 16:30, peperivera2652738
In chile, the deepest earthquake occurred at 61.7°w longitude at a depth of 540 km. if the rocks at the focus began subducting 10 million years ago and are now 1000 km from their original position, what is the average rate of subduction in cm/yr?
Answers: 1

Chemistry, 23.06.2019 19:30, FantasticFerret
What is the pressure of 5.0 mol nitrogen gas in a 2.0 l container at 268 k (the universal gas constant is 0.0821 l•atm/mol•k) a. 55 atm b. 8.8 atm c. 0.018 atm d. 220 atm
Answers: 1

Chemistry, 24.06.2019 02:00, hollandhogenson
Using henry's law, calculate the molar concentration of o2 in the surface water of a mountain lake saturated with air at 20 ∘c and an atmospheric pressure of 685 torr .
Answers: 1
You know the right answer?
In lab, a student burns 0.550 grams of Ritz crackers. The burning causes 100.0 grams of water to ris...
Questions in other subjects:


Mathematics, 25.02.2021 18:40


Biology, 25.02.2021 18:40




Mathematics, 25.02.2021 18:40

Mathematics, 25.02.2021 18:40

Mathematics, 25.02.2021 18:40