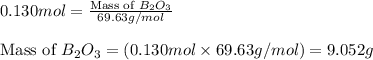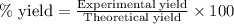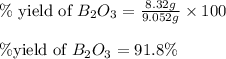Chemistry, 29.03.2020 21:45 demarpratt1270
Find percent yield:
The mass of B2O3 produced by the reaction of 4.00 g of B5H9, and 10.00 g of
O2 is 8.32 g. What is the percent yield?
2 B3H9 + 12 O2 => 5 B2O3 +9 H2O
87.2
92.8
91.8
75.5
74.5

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, penelopegrace04
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 21.06.2019 18:00, anamaliiow
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
Find percent yield:
The mass of B2O3 produced by the reaction of 4.00 g of B5H9, and 10....
The mass of B2O3 produced by the reaction of 4.00 g of B5H9, and 10....
Questions in other subjects:

Mathematics, 04.03.2021 17:20


Business, 04.03.2021 17:20

Mathematics, 04.03.2021 17:20

History, 04.03.2021 17:20


Mathematics, 04.03.2021 17:20


Mathematics, 04.03.2021 17:20

 .....(1)
.....(1)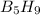 :
: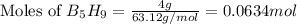
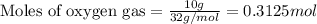
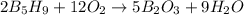
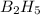
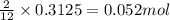 of
of 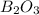
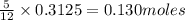 of water
of water