

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 23.06.2019 18:30, johncena1001
Esure to answer all parts. the equilibrium constant for the reaction ni2+(aq) + 6 nh3(aq) ⇌ ni(nh3)6 2+(aq) is kf = 5.6 × 108 at 25°c. (a) what is δg o at this temperature? (b) if standard-state concentrations of reactants and products are mixed, in which direction does the reaction proceed? (c) determine δg when [ni(nh3)62+] = 0.010 m, [ni2+] = 0.0010 m, and [nh3] = 0.0050 m. in which direction will the reaction proceed to achieve equilibrium? (a) × 10 j/mol (enter your answer in scientific notation.) (b) to the right. to the left. (c) × 10 j/mol (enter your answer in scientific notation.) to the right. to the left.
Answers: 3

Chemistry, 23.06.2019 20:00, adalan6986
Assume you inhale in about 6 l of air per minute how much nitrogen do you breathe in one minute
Answers: 1

Chemistry, 23.06.2019 21:30, peachijmin
Acompound containing nitrogen and oxygen is decomposed in the laboratory and produces 1.78 g of nitrogen and 4.05 g of oxygen. part a calculate the empirical formula of the compound.
Answers: 1
You know the right answer?
How many moles of CO2 will be produced from 97.0 g of C3 Hy, assuming O2 is available in excess?...
Questions in other subjects:


Mathematics, 18.11.2020 18:50








Mathematics, 18.11.2020 18:50

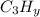 is 3 moles: that is so because every molecule of
is 3 moles: that is so because every molecule of 

