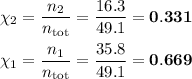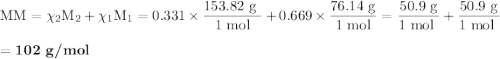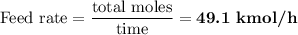Chemistry, 29.03.2020 04:06 zoeedadoll
A solution of carbon tetrachloride and carbon disulfide containing 50% by weight of each is to be continuously distilled at a rate of 5000 kg/h.
(a) Determine the concentration of the mixture in terms of mole fractions. (b) Determine the average molecular weight of the mixture.
(c) Calculate the feed rate in kmol/h.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

You know the right answer?
A solution of carbon tetrachloride and carbon disulfide containing 50% by weight of each is to be co...
Questions in other subjects:

Computers and Technology, 20.02.2020 08:00


Computers and Technology, 20.02.2020 08:00





Social Studies, 20.02.2020 08:00