
Chemistry, 28.03.2020 22:53 ExperimentsDIYS
Initially the concentration of H2 is 2.0M , I2 is 2.0M and HI is 8.0M. The vessel is heated up to 675 degrees at equilibrium the concentration of HI changes to become 6.0M. Calculate the value of k at this temperature.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2


Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Initially the concentration of H2 is 2.0M , I2 is 2.0M and HI is 8.0M. The vessel is heated up to 67...
Questions in other subjects:

Mathematics, 16.02.2021 08:10


Mathematics, 16.02.2021 08:10

Mathematics, 16.02.2021 08:10




Mathematics, 16.02.2021 08:10

Chemistry, 16.02.2021 08:10

![\frac{[HI]^{2} }{[H]_{2} I_{2} }](/tpl/images/0569/1352/e5ad7.png)
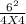
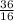 = 2.25
= 2.25

