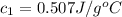Chemistry, 28.03.2020 17:59 meiyrarodriguez
A 24.87 gram sample of a metal at 104.0ºC was added to 76.12 grams of water at 25.2ºC in a perfectly insulated calorimeter. The final temperature of the metal-water mixture was 28.2ºC. Calculate the specific heat capacity of the metal using the data. Type your work and answer below. Make sure to include a unit on the final answer.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
A 24.87 gram sample of a metal at 104.0ºC was added to 76.12 grams of water at 25.2ºC in a perfectly...
Questions in other subjects:

Mathematics, 11.10.2019 17:30

Social Studies, 11.10.2019 17:30

Mathematics, 11.10.2019 17:30

Biology, 11.10.2019 17:30

History, 11.10.2019 17:30



Mathematics, 11.10.2019 17:30


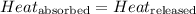
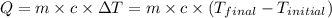
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0568/9675/09236.png) ......(1)
......(1)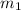 = mass of metal = 24.87 g
= mass of metal = 24.87 g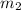 = mass of water = 76.12 g
= mass of water = 76.12 g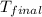 = final temperature = 28.2°C
= final temperature = 28.2°C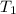 = initial temperature of metal = 104.0°C
= initial temperature of metal = 104.0°C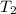 = initial temperature of water = 25.2°C
= initial temperature of water = 25.2°C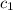 = specific heat of metal = ?
= specific heat of metal = ? = specific heat of water = 4.186 J/g°C
= specific heat of water = 4.186 J/g°C![24.87\times c_1\times (28.2-104)=-[76.12\times 4.186\times (28.2-25.2)]](/tpl/images/0568/9675/a6f4b.png)