What is the equilibrium constant expression for the given system?
k subscript e q equals Start...

What is the equilibrium constant expression for the given system?
k subscript e q equals StartFraction StartBracket upper H subscript 2 upper O EndBracket over StartBracket upper H subscript 2 EndBracket StartBracket upper O subscript 2 EndBracket EndFraction.
k subscript e q equals StartFraction StartBracket upper H subscript 2 upper O EndBracket superscript 2 over StartBracket upper H subscript 2 EndBracket superscript 2 StartBracket upper O subscript 2 EndBracket EndFraction.
K subscript e q equals StartFraction StartBracket upper H subscript 2 EndBracket superscript 2 StartBracket upper O subscript 2 EndBracket over StartBracket upper H subscript 2 upper O EndBracket EndFraction.
K subscript e q equals StartFraction StartBracket upper H subscript 2 EndBracket superscript 2 StartBracket upper O subscript 2 EndBracket over StartBracket upper H subscript 2 upper O EndBracket superscript 2 EndFraction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1


You know the right answer?
Questions in other subjects:

Mathematics, 26.02.2021 20:30

Social Studies, 26.02.2021 20:30


Mathematics, 26.02.2021 20:30

Mathematics, 26.02.2021 20:30


Mathematics, 26.02.2021 20:30


Mathematics, 26.02.2021 20:30

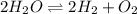
![K_{eq}=\frac{[H_2]^2[O_2]}{[H_2O]^2}](/tpl/images/0568/8225/1f40d.png)
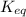
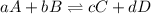
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0568/8225/9c8b0.png)



