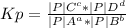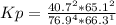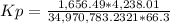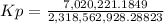Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2(g) → 2 Cl2(g) + 2 H2O(g)
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen chloride, oxygen, chlorine, and water has the following composition:
COMPOUND Pressure at equilibrium
HCl 76.9 atm
O2 66.3 atm
Cl2 40.7 atm
H2O 65.1 atm
Calculate the value of the equilibrium constant Kp for this reaction. Round your answer to 2 significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:10, ChloeLiz7111
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2...
4 HCl(g) + O2...
Questions in other subjects:

Mathematics, 10.02.2020 23:55

Biology, 10.02.2020 23:55

Mathematics, 10.02.2020 23:55


Mathematics, 10.02.2020 23:55


Mathematics, 10.02.2020 23:55