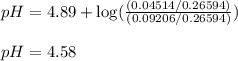Chemistry, 28.03.2020 04:02 cupcake20019peehui
An analytical chemist is titrating of a solution of propionic acid with a solution of 224.9 ml of a 0.6100M solution of propionic acid (HC2H5CO2) with a 1.1000M solution of KOH. The pKa of proionic acid 4.89.
Calculate the pH of the acid solution after the chemist has added 41.04mL of the KOH solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to decimal places.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, soliseric879
Dwayne filled a small balloon with air at 298.5 k. he put the balloon into a bucket of water, and the water level in the bucket increased by 0.54 liter. if dwayne puts the balloon into a bucket of ice water at 273.15 k and waits for the air inside the balloon come to the same temperature, what will the volume of the balloon be? assume the pressure inside the balloon doesn’t change. type the correct answer in the box. express your answer to the correct number of significant figures. the volume of the balloon at 273.15 k is liters.
Answers: 2



Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
You know the right answer?
An analytical chemist is titrating of a solution of propionic acid with a solution of 224.9 ml of a...
Questions in other subjects:

English, 26.02.2021 22:30


Mathematics, 26.02.2021 22:30

History, 26.02.2021 22:30


Mathematics, 26.02.2021 22:30

Mathematics, 26.02.2021 22:30

Mathematics, 26.02.2021 22:30


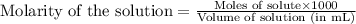 .....(1)
.....(1)
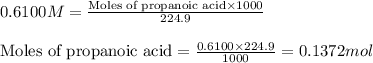
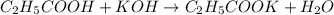
![pH=pK_a+\log(\frac{[\text{salt}]}{[acid]})](/tpl/images/0568/6689/3d096.png)
![pH=pK_a+\log(\frac{[C_2H_5COOK]}{[C_2H_5COOH]})](/tpl/images/0568/6689/3ede9.png)
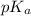 = negative logarithm of acid dissociation constant of propanoic acid = 4.89
= negative logarithm of acid dissociation constant of propanoic acid = 4.89![[C_2H_5COOK]=\frac{0.04514}{0.26594}](/tpl/images/0568/6689/11196.png)
![[C_2H_5COOH]=\frac{0.09206}{0.26594}](/tpl/images/0568/6689/86178.png)