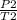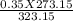Gay-Lussac Problems
P./T1 = P2/T2 - Remember, T is in KELVIN, add 273 to any temp given to you in °C to convert
1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0
°C to 30.0 °C.
2. If a gas in a closed container is pressurized from 15.0 atmospheres to 16.0 atmospheres and its
original temperature was 25.0 °C, what would the final temperature of the gas be?
3. A gas has a pressure of 0.35 atm at 50.0 °C. What is the pressure at standard temperature?
Boyle's law Drobleme

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:20, zymikaa00
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 06:30, themajesty9898
The minerals found in bones are deposited by living cells called
Answers: 1
You know the right answer?
Gay-Lussac Problems
P./T1 = P2/T2 - Remember, T is in KELVIN, add 273 to any temp given to you...
P./T1 = P2/T2 - Remember, T is in KELVIN, add 273 to any temp given to you...
Questions in other subjects:


Computers and Technology, 08.10.2019 07:00



Chemistry, 08.10.2019 07:00



Computers and Technology, 08.10.2019 07:00


Mathematics, 08.10.2019 07:00

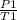 =
=