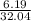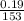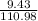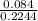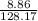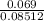Chemistry, 27.03.2020 19:31 carlosleblanc26
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methanol (CH3OH) in 1.50 × 102 mL of solution:
mol/L
(b) 9.43 g of calcium chloride (CaCl2) in 2.20 × 102 mL of solution:
mol/L
(c) 8.86 g of naphthalene (C10H8) in 85.2 mL of benzene solution:
mol/L

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1


Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methano...
(a) 6.19 g of methano...
Questions in other subjects:








History, 12.06.2020 22:57


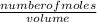 equation 1
equation 1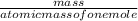 equation 2
equation 2