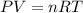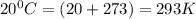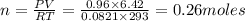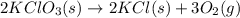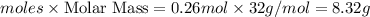Oxygen gas can be prepared by heating potassium chlorate according to the following equation:
<...

Oxygen gas can be prepared by heating potassium chlorate according to the following equation:
2KClO3(s) → 2KCl(s) + 3O2(g)
The product gas, O2, is collected over water at a temperature of 20 °C and a pressure of 747 mm Hg. If the wet O2 gas formed occupies a volume of 6.42 L, the number of grams of O2 formed is g. The vapor pressure of water is 17.5 mm Hg at 20 °C.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 23.06.2019 00:30, zaniathomasel
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 05:30, zeesharpe05
If c + di is a point on the circle, then | c + di |=
Answers: 2

Chemistry, 23.06.2019 13:30, gavianacandelar8522
The process by which liquid water changes into water vapour is called what ?
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 13.10.2020 15:01



Chemistry, 13.10.2020 15:01

English, 13.10.2020 15:01




English, 13.10.2020 15:01

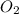 formed is 8.32 g
formed is 8.32 g