
Chemistry, 27.03.2020 16:45 janahiac09
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?
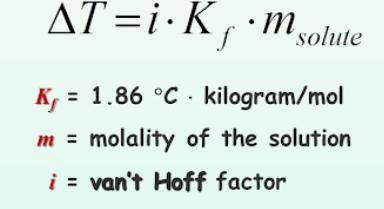

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2
You know the right answer?
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?<...
Questions in other subjects:




Mathematics, 19.12.2019 15:31


English, 19.12.2019 15:31

Mathematics, 19.12.2019 15:31

Mathematics, 19.12.2019 15:31

English, 19.12.2019 15:31

History, 19.12.2019 15:31



