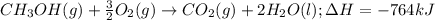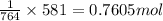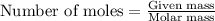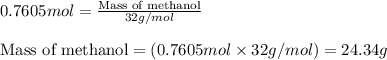Chemistry, 27.03.2020 02:17 austin8535
When methanol, CH 3 OH , is burned in the presence of oxygen gas, O 2 , a large amount of heat energy is released. For this reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH 3 OH ( g ) + 3 2 O 2 ( g ) ⟶ CO 2 ( g ) + 2 H 2 O ( l ) Δ H = − 764 kJ How much methanol, in grams, must be burned to produce 581 kJ of heat?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

You know the right answer?
When methanol, CH 3 OH , is burned in the presence of oxygen gas, O 2 , a large amount of heat energ...
Questions in other subjects:

Geography, 02.09.2019 00:30


Social Studies, 02.09.2019 00:30




English, 02.09.2019 00:30


History, 02.09.2019 00:30