
Chemistry, 27.03.2020 01:50 marsewilliams
Suppose the reaction system below has already reached equilibrium. Predict the effect that each of the following changes will have on the equilibrium position. Tell whether the equilibrium will shift to the right, will shift to the left, or will not be affected.
UO2(s) + 4 HF(g) ↔ UF4(g) + 2 H2O(g)
1. Water vapor is removed
2. UF4(g) is added
3. The reaction is done in a glass reaction vessel. HF(g) attacks and reacts with the glass
4. If the reaction is endothermic, and you want to make the equilibrium shift to the right to maximize the products, would you heat or cool the reaction vessel?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Suppose the reaction system below has already reached equilibrium. Predict the effect that each of t...
Questions in other subjects:


Mathematics, 09.01.2021 08:20


Mathematics, 09.01.2021 08:20



English, 09.01.2021 08:20

Mathematics, 09.01.2021 08:20

Physics, 09.01.2021 08:20

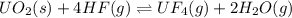
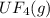 is added
is added

