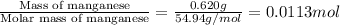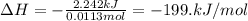Chemistry, 27.03.2020 00:04 cgarnett5408
When 0.620 gMngMn is combined with enough hydrochloric acid to make 100.0 mLmL of solution in a coffee-cup calorimeter, all of the MnMn reacts, raising the temperature of the solution from 23.1 ∘C∘C to 28.9 ∘C∘C. Find ΔHrxnΔHrxn for the reaction as written. (Assume that the specific heat capacity of the solution is 4.18 J/g∘CJ/g∘C and the density is 1.00 g/mLg/mL.)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
You know the right answer?
When 0.620 gMngMn is combined with enough hydrochloric acid to make 100.0 mLmL of solution in a coff...
Questions in other subjects:

Mathematics, 31.07.2020 20:01






History, 31.07.2020 20:01

Mathematics, 31.07.2020 20:01


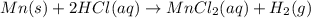
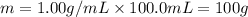
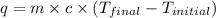
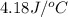
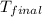 = final temperature =
= final temperature = 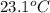
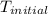 = initial temperature =
= initial temperature = 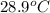
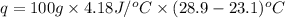
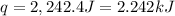
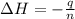
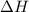 = enthalpy change = ?
= enthalpy change = ?