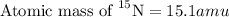The element nitrogen has an atomic weight of 14.0 and consists of two stable isotopes nitrogen-14 and nitrogen-15. The isotope nitrogen-14 has a mass of 14.0 amu and a percent natural abundance of 99.6 %. The isotope nitrogen-15 has a percent natural abundance of 0.370 %. What is the mass of nitrogen-15? amu

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3


Chemistry, 22.06.2019 22:30, brianna5626
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
The element nitrogen has an atomic weight of 14.0 and consists of two stable isotopes nitrogen-14 an...
Questions in other subjects:


History, 22.05.2020 07:04


Mathematics, 22.05.2020 07:04

Mathematics, 22.05.2020 07:04



Mathematics, 22.05.2020 07:04


Social Studies, 22.05.2020 07:04

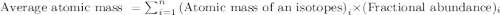 .....(1)
.....(1)
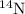 isotope:
isotope: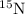 isotope:
isotope:![14.0=[(14.0\times 0.996)+(\text{Atomic mass of }^{15}\textrm{N}\times 0.00370)]](/tpl/images/0566/2813/d6364.png)