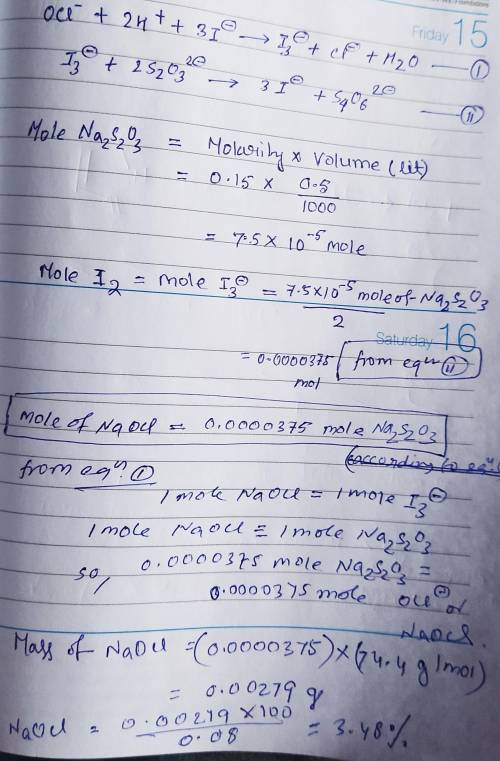Chemistry, 26.03.2020 23:44 taylord4230
In part 2 of the experiment, you will be analyzing a sample of household bleach. A 0.0800 g sample of household bleach is completely reacted with KI(s). The resulting solution is then titrated with 0.150 M NaS2O3 solution. 0.500 mL of the solution is required to reach the colorless endpoint. What is the mass percent of NaOCl (MM = 74.44 g/mole) in the bleach?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, annafellows
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3


Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
In part 2 of the experiment, you will be analyzing a sample of household bleach. A 0.0800 g sample o...
Questions in other subjects:




History, 22.03.2021 15:40

Chemistry, 22.03.2021 15:40




Mathematics, 22.03.2021 15:40