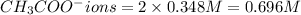In the laboratory you dissolve 14.8 g of chromium(II) acetate in a volumetric flask and add water to a total volume of 250 mL. What is the molarity of the solution? M. What is the concentration of the chromium(II) cation? M. What is the concentration of the acetate anion? M. Submit AnswerRetry Entire Group

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1


You know the right answer?
In the laboratory you dissolve 14.8 g of chromium(II) acetate in a volumetric flask and add water to...
Questions in other subjects:





Mathematics, 03.12.2021 01:00


Business, 03.12.2021 01:00



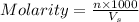
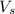 = volume of solution in ml = 150 ml
= volume of solution in ml = 150 ml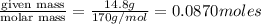
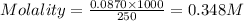
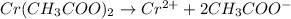
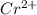 ions = 0.348 M
ions = 0.348 M