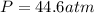Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
You know the right answer?
A 1.93-mol sample of xenon gas is maintained in a 0.805-L container at 306 K. Calculate the pressure...
Questions in other subjects:




Mathematics, 16.07.2021 04:30


Mathematics, 16.07.2021 04:30


Biology, 16.07.2021 04:30

Chemistry, 16.07.2021 04:30

Mathematics, 16.07.2021 04:30

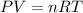
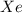 gas = ?
gas = ?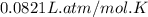
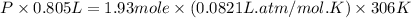
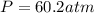
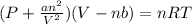
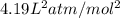
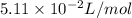
![(P+\frac{(4.19L^2atm/mol^2)\times (1.93mole)^2}{(0.805L)^2})[0.805L-(1.93mole)\times (5.11\times 10^{-2}L/mol)]=1.93mole\times (0.0821L.atm/mol.K)\times 306K](/tpl/images/0566/1070/6fd78.png)