
Chemistry, 26.03.2020 22:48 giraffesaur44
Determine the expression for the rate of the reaction with respect to each of the reactants and products. Determine the expression for the rate of the reaction with respect to each of the reactants and products. Rate=−13Δ[A]Δt=−Δ[B]Δt=12Δ[C]ΔtRate =−13Δ[A]Δt=−Δ[B]Δt=12Δ[C]Δt Rate=−12Δ[A]Δt=−Δ[B]Δt=13Δ[C]ΔtRate =−12Δ[A]Δt=−Δ[B]Δt=13Δ[C]Δt Rate=−Δ[A]Δt=−12Δ[B]Δt=13Δ[C]ΔtRate =−Δ[A]Δt=−12Δ[B]Δt=13Δ[C]Δt Rate=12Δ[A]Δt=12Δ[B]Δt=13Δ[C]Δt

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, angelteddy033
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2



Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
Determine the expression for the rate of the reaction with respect to each of the reactants and prod...
Questions in other subjects:




Physics, 27.05.2021 14:10

English, 27.05.2021 14:10

Law, 27.05.2021 14:10

English, 27.05.2021 14:10

Social Studies, 27.05.2021 14:10

Social Studies, 27.05.2021 14:10

Mathematics, 27.05.2021 14:10

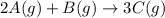
![Rate=-\frac{1}{3}\frac{\Delta [A]}{\Delta t}=-\frac{\Delta [B]}{\Delta t}=\frac{1}{2}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/ff0f8.png)
![Rate=-\frac{1}{2}\frac{\Delta [A]}{\Delta t}=-\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/11b91.png)
![Rate=-\frac{\Delta [A]}{\Delta t}=-\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/f7a0b.png)
![Rate=\frac{1}{2}\frac{\Delta [A]}{\Delta t}=\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/3152a.png)
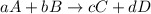
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0566/1120/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0566/1120/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0566/1120/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0566/1120/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0566/1120/d4b94.png)
![\text{Rate of disappearance of }A=-\frac{1}{2}\frac{\Delta [A]}{\Delta t}](/tpl/images/0566/1120/4cd85.png)
![\text{Rate of disappearance of }B=-\frac{\Delta [B]}{\Delta t}](/tpl/images/0566/1120/43240.png)
![\text{Rate of formation of }C=+\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/d1447.png)


