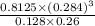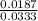Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 04:00, mgnbrnne
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
You know the right answer?
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous CH4 and H2...
Questions in other subjects:



Mathematics, 08.12.2020 08:20

Biology, 08.12.2020 08:20

Social Studies, 08.12.2020 08:20

English, 08.12.2020 08:20

Chemistry, 08.12.2020 08:20

Mathematics, 08.12.2020 08:20

Mathematics, 08.12.2020 08:20

![[H_{2}O]](/tpl/images/0566/0391/04475.png) at equilibrium is 0.561 M.
at equilibrium is 0.561 M.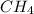 = 0.041 mol,
= 0.041 mol,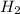 = 0.091 mol
= 0.091 mol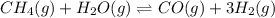
![\frac{[CO][H_{2}]^{3}}{[CH_{4}][H_{2}O]}](/tpl/images/0566/0391/7f129.png) ...... (1)
...... (1)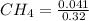 = 0.128 M,
= 0.128 M,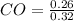 = 0.8125 M,
= 0.8125 M, 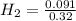 = 0.284 M
= 0.284 M![[H_{2}O] = \frac{[CO][H_{2}]^{3}}{[CH_{4}] \times K}](/tpl/images/0566/0391/7448a.png)