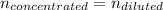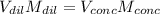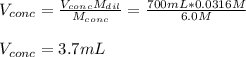Chemistry, 26.03.2020 21:52 MathChic68
A chemist must prepare 700.0mL of nitric acid solution with a pH of 1.50 at 25°C. He will do this in three steps: Fill a 700.0mL volumetric flask about halfway with distilled water. Measure out a small volume of concentrated (6.0M) stock nitric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated nitric acid that the chemist must measure out in the second step. Round your answer to 2 significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
A chemist must prepare 700.0mL of nitric acid solution with a pH of 1.50 at 25°C. He will do this in...
Questions in other subjects:



Mathematics, 02.03.2021 16:10

Advanced Placement (AP), 02.03.2021 16:10

Mathematics, 02.03.2021 16:10

Arts, 02.03.2021 16:10


Mathematics, 02.03.2021 16:10

Chemistry, 02.03.2021 16:10

Mathematics, 02.03.2021 16:10


![[H]^+=10^{-pH}=10^{-1.50}=0.0316M](/tpl/images/0565/8791/85748.png)
![[H]^+=[HNO_3]=0.0316M](/tpl/images/0565/8791/de553.png)