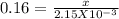The value of Δ G ° ' ΔG°′ for the conversion of 3-phosphoglycerate to 2-phosphoglycerate (2PG) is + 4.40 kJ/mol +4.40 kJ/mol . If the concentration of 3-phosphoglycerate at equilibrium is 2.15 mM 2.15 mM , what is the concentration of 2-phosphoglycerate? Assume a temperature of 25.0 ° C 25.0°C .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1


Chemistry, 23.06.2019 08:00, IntellTanito
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
The value of Δ G ° ' ΔG°′ for the conversion of 3-phosphoglycerate to 2-phosphoglycerate (2PG) is +...
Questions in other subjects:


English, 11.03.2021 09:00

Mathematics, 11.03.2021 09:00

Chemistry, 11.03.2021 09:00


Advanced Placement (AP), 11.03.2021 09:10



Social Studies, 11.03.2021 09:10

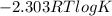
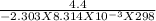
![K = \frac{[2 - phosphogycerate]}{[3-phosphoglycerate]}](/tpl/images/0565/8684/ed7d4.png)