
Chemistry, 26.03.2020 22:01 kharmaculpepper
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) 2.22 × 10-2 M/s 1.11 × 10-1 M/s 4.44 × 10-2 M/s 8.88 × 10-2 M/s 5.25 × 10-2 M/s

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1


Chemistry, 23.06.2019 06:00, mustafajibawi1
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

Chemistry, 23.06.2019 06:00, kristine2424
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the...
Questions in other subjects:

Physics, 02.10.2019 17:10

Mathematics, 02.10.2019 17:10


History, 02.10.2019 17:10

Mathematics, 02.10.2019 17:10

Social Studies, 02.10.2019 17:10


Mathematics, 02.10.2019 17:10

English, 02.10.2019 17:10

History, 02.10.2019 17:10

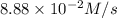
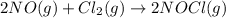
![-\frac{1d[NO]}{2dt}=-\frac{d[Cl_2]}{dt}=+\frac{1d[NOCl]}{2dt}](/tpl/images/0565/9272/04d4d.png)
![\frac{-d[Cl_2]}{dt}]=4.44\times 10^{-2}M/s](/tpl/images/0565/9272/271c7.png)
![-\frac{1d[NO]}{dt}=2\times {\frac{-d[Cl_2]}{dt}=2\times 4.44\times 10^{-2}M/s=8.88\times 10^{-2}M/s](/tpl/images/0565/9272/dea34.png)


