
Chemistry, 26.03.2020 21:35 CHENDESHEN
A compound is found to contain 9.224 % boron and 90.74 % chlorine by mass. To answer the question, enter the elements in the order presented above. QUESTION 1: The empirical formula for this compound is . QUESTION 2: The molar mass for this compound is 117.2 g/mol. The molecular formula for this compound is .

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 21.06.2019 23:50, kukisbae
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1


Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3
You know the right answer?
A compound is found to contain 9.224 % boron and 90.74 % chlorine by mass. To answer the question, e...
Questions in other subjects:



Biology, 09.01.2021 02:40

English, 09.01.2021 02:40


Mathematics, 09.01.2021 02:40

Mathematics, 09.01.2021 02:40

History, 09.01.2021 02:40

Mathematics, 09.01.2021 02:40

Mathematics, 09.01.2021 02:40

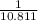 =0.85 mol (Mass value of boron=10.811)
=0.85 mol (Mass value of boron=10.811)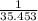 =2.559 mol.(Mass value of chlorine=35.453)
=2.559 mol.(Mass value of chlorine=35.453)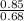 =1
=1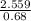 =3
=3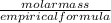
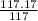 ≈1
≈1

