
Chemistry, 26.03.2020 20:57 zhellyyyyy
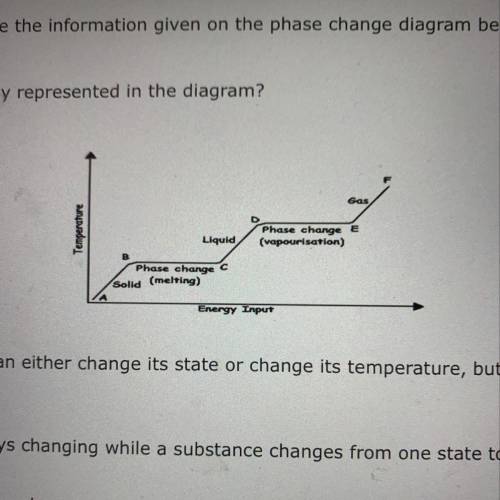
Which statement represents this diagram? A. A given substance can either change its state or change its temperature, but not both at the same
time.
B. Temperature is always changing while a substance changes from one state to another.
C. A given substance can change its state at any temperature.
D. The energy being added can be used to change the temperature or the state of matter at the same
time.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, Gghbhgy4809
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1


Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 13:30, querline87
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1
You know the right answer?
Which statement represents this diagram? A. A given substance can either change its state or change...
Questions in other subjects:

Mathematics, 25.01.2021 16:30

Computers and Technology, 25.01.2021 16:30

Mathematics, 25.01.2021 16:30

English, 25.01.2021 16:30


History, 25.01.2021 16:30



Mathematics, 25.01.2021 16:30

Social Studies, 25.01.2021 16:30



