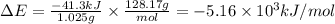Chemistry, 26.03.2020 20:54 Gearyjames8
Mothballs are composed primarily of naphthalene (C10H8). When 1.025 g of naphthalene burns in a bomb calorimeter, the temperature rises from 24.25 C to 32.33 C. (The Heat Capacity of the Calorimeter is 5.11 kJ/C.) Find DeltaE (in x 103 kJ) for the combustion of naphthalene.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 23.06.2019 01:30, Dmoney5104
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
Mothballs are composed primarily of naphthalene (C10H8). When 1.025 g of naphthalene burns in a bomb...
Questions in other subjects:


English, 01.02.2021 16:10




English, 01.02.2021 16:20


Spanish, 01.02.2021 16:20

Chemistry, 01.02.2021 16:20