Chemistry, 26.03.2020 20:31 sparky1234
1. Tooth enamel consists mainly of hydroxyapatite (Ca5(PO4)3(OH). When tin(II)fluoride is added to toothpaste it reacts with the enamel to produce a more decay-resistant material fluoroapatite ( Ca5(PO4)3F. The by-products of this reaction are tin(II)oxide and water. What mass of hydroxyapatite can be converted to fluoroapatite by reaction with 0.115 grams of tin(II)fluoride

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
1. Tooth enamel consists mainly of hydroxyapatite (Ca5(PO4)3(OH). When tin(II)fluoride is added to t...
Questions in other subjects:


Mathematics, 30.08.2019 03:30

Spanish, 30.08.2019 03:30

Mathematics, 30.08.2019 03:30





Mathematics, 30.08.2019 03:30

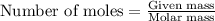 .....(1)
.....(1)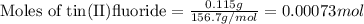
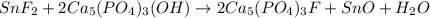
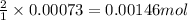 of hydroxyapatite
of hydroxyapatite