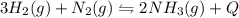In the reaction represented by the equation n2(g) + 3h2(g) 2nh3(g), the
forward reaction is ex...

Chemistry, 10.10.2019 16:30 jacobbecker99
In the reaction represented by the equation n2(g) + 3h2(g) 2nh3(g), the
forward reaction is exothermic. which set of conditions would give the best yield of
ammonia at equilibrium?
a) 800 atmospheres and 2000 oc
b) 1 atmosphere and 500 o
c
c) 1 atmosphere and 2000 o
c
d) 800 atmospheres and 500 o
c

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 12.05.2021 19:10

Mathematics, 12.05.2021 19:10

Social Studies, 12.05.2021 19:10


Social Studies, 12.05.2021 19:10


History, 12.05.2021 19:10