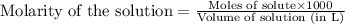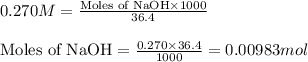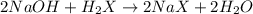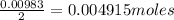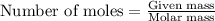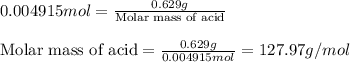A 0.629 g sample of a diprotic acid is dissolved in water and titrated with 0.270 M NaOH. What is the molar mass of the acid if 36.4 mL of the NaOH solution is required to neutralize the sample? Assume the volume of NaOH corresponds to the second equivalence point. A flask with a solution sits on the base of a ring stand. A buret filled with liquid is suspended above the flask by the ring stand. molar mass: g/mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
A 0.629 g sample of a diprotic acid is dissolved in water and titrated with 0.270 M NaOH. What is th...
Questions in other subjects:



English, 25.02.2021 22:50


Chemistry, 25.02.2021 22:50

Mathematics, 25.02.2021 22:50

Mathematics, 25.02.2021 22:50