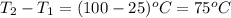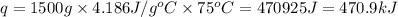How much heat is required to warm 1.50L of water from 25.0C to 100.0C? (Assume a density of 1.0g/mL for the water.)
My brain wants to just start with 1.5L and use density as a conversion factor but I seriously think I'm missing something. I don't really understand the heat equation, but I'm thinking I might need to use q = mCAT?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, arodavoarodavo
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
How much heat is required to warm 1.50L of water from 25.0C to 100.0C? (Assume a density of 1.0g/mL...
Questions in other subjects:



Mathematics, 11.03.2021 03:00

Mathematics, 11.03.2021 03:00


Arts, 11.03.2021 03:00




Mathematics, 11.03.2021 03:00

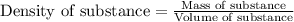
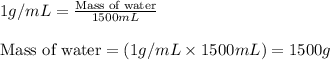
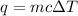
 = change in temperature =
= change in temperature =