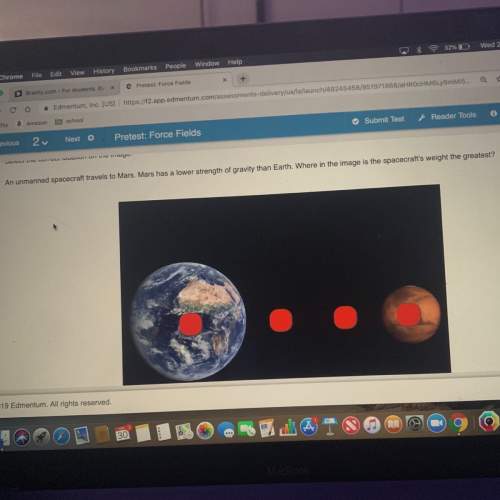
Chemistry, 26.03.2020 16:47 caldonia2018
If the reaction below takes place at STP how many L of ammonia (NH3) will be formed if one starts with 3.45 moles of hydrogen and an excess of nitrogen? Be sure to balance the equation before you begin and round to the proper accuracy. Please leave any units off.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

You know the right answer?
If the reaction below takes place at STP how many L of ammonia (NH3) will be formed if one starts wi...
Questions in other subjects:


Mathematics, 06.03.2020 13:54



Medicine, 06.03.2020 13:55

Mathematics, 06.03.2020 13:55


English, 06.03.2020 13:56


Biology, 06.03.2020 13:56