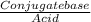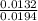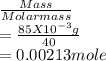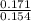Chemistry, 26.03.2020 05:19 kkjones1536
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. Part A: What is the initial pH of this solution?Part B: What is the pH after addition of 150.0 mg of HBr?Part C: What is the pH after addition of 85.0 mg of NaOH?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, emilyborland50
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d. the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2


Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1
You know the right answer?
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. Part A: What is the initial pH o...
Questions in other subjects:

Mathematics, 04.04.2020 06:07




English, 04.04.2020 06:07





![\frac{[Conjugate base]}{[Acid]}](/tpl/images/0564/8323/225c1.png)
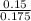
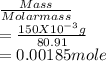
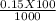 = 0.015
= 0.015 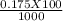 = 0.0175
= 0.0175