

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
The first-order isomerization reaction: cyclopropane → propene has a rate constant of 1.10×10-4 s-1...
Questions in other subjects:


Business, 20.05.2021 17:20

Biology, 20.05.2021 17:20


Computers and Technology, 20.05.2021 17:30


Mathematics, 20.05.2021 17:30


Mathematics, 20.05.2021 17:30

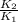 =
= 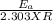
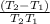
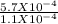 =
= 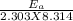
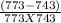
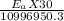
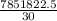 j/mole = 261.7 Kj/mole
j/mole = 261.7 Kj/mole

