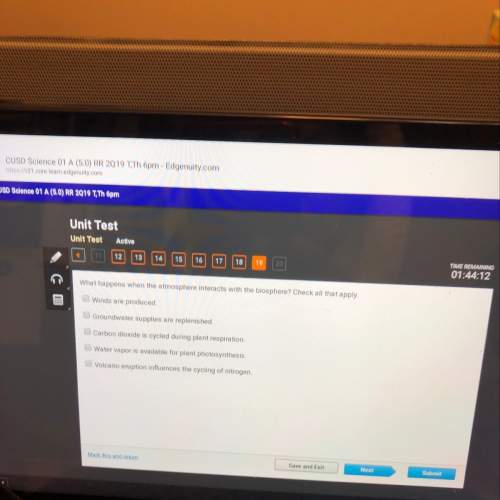
Chemistry, 25.03.2020 23:15 ambriaramirez8
You are given aqueous solutions of six different substances and asked to determine whether they are
strong, weak, or nonelectrolytes. Describe how you could test the solutions. Include definitions of strong
electrolyte, weak electrolyte, and nonelectrolyte in your answer and explain how your test would allow
you to differentiate between the solutions.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
You are given aqueous solutions of six different substances and asked to determine whether they are<...
Questions in other subjects:

English, 17.11.2020 20:20

Mathematics, 17.11.2020 20:20


Mathematics, 17.11.2020 20:20

Chemistry, 17.11.2020 20:20

Mathematics, 17.11.2020 20:20

Mathematics, 17.11.2020 20:20

Biology, 17.11.2020 20:20


Mathematics, 17.11.2020 20:20