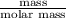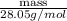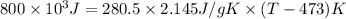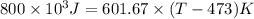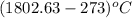Chemistry, 25.03.2020 20:21 bvargas786p7aa8y
For steady flow through heat exchanger at approximately atmospheric pressure, what is the final temperature when heat in the amount of 800 kJ is added to 10 moles of ethylene initially at 200 °C?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2


You know the right answer?
For steady flow through heat exchanger at approximately atmospheric pressure, what is the final temp...
Questions in other subjects:


Computers and Technology, 01.07.2019 22:10







History, 01.07.2019 22:10

Social Studies, 01.07.2019 22:10

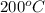 is
is 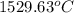 .
.