
Chemistry, 25.03.2020 20:19 ashley232323
Given the balanced equation representing a reaction:
2HCl(aq) + Na2S2O3(aq)--> S(s) + H2SO3(aq) + 2NaCl(aq)
Decreasing the concentration of Na2S2O3(aq) decreases the rate of reaction
because the
(1) activation energy decreases
(2) activation energy increases
(3) frequency of effective collisions decreases
(4) frequency of effective collisions increases

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2


You know the right answer?
Given the balanced equation representing a reaction:
2HCl(aq) + Na2S2O3(aq)--> S(s) +...
2HCl(aq) + Na2S2O3(aq)--> S(s) +...
Questions in other subjects:



Mathematics, 04.12.2020 07:20

Mathematics, 04.12.2020 07:20

Biology, 04.12.2020 07:20


Mathematics, 04.12.2020 07:20

Mathematics, 04.12.2020 07:20


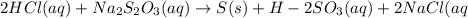
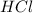 and
and  in the given case.
in the given case.

