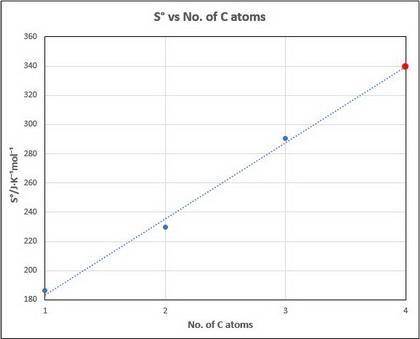
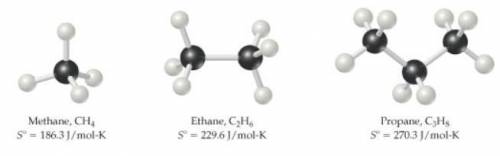
What might you expect for the value of S∘ (entropy) for butane, C4H10?
Entropies provided (Hin...

Chemistry, 25.03.2020 20:08 shaylawaldo11
What might you expect for the value of S∘ (entropy) for butane, C4H10?
Entropies provided (Hint: entropy increases with increasing molecular complexity):
- CH4=186.3(J/mol*K)
-C2H6=229.6(J/mol*K)
-C2H8=270.3(J/mol*K)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, ayahabdulhaqq2
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 21.06.2019 23:50, ShlomoShekelstein
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 19:40, powberier6979
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 08.09.2020 05:01

Mathematics, 08.09.2020 05:01

Mathematics, 08.09.2020 05:01

Biology, 08.09.2020 05:01

Mathematics, 08.09.2020 05:01

Mathematics, 08.09.2020 05:01

Mathematics, 08.09.2020 05:01


Mathematics, 08.09.2020 05:01