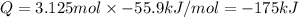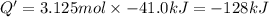125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The heat of neutralization for the formation of water -55.9 kJ/mole. The heat of solvation (it might be called the heat of solution) for sodium hydroxide is 41.0 kJ/mole What quantity of heat is released?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:10, lilyjordan5972
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The hea...
Questions in other subjects:

Mathematics, 15.01.2021 23:00

Mathematics, 15.01.2021 23:00

Chemistry, 15.01.2021 23:00

Social Studies, 15.01.2021 23:00


Mathematics, 15.01.2021 23:00


Chemistry, 15.01.2021 23:00


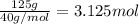

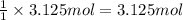 of water
of water