
Chemistry, 25.03.2020 16:17 jimarieb08
In Part A, you found the number of moles of product (1.80 mol P2O5 ) formed from the given amount of phosphorus and excess oxygen. In Part B, you found the number of moles of product (1.40 mol P2O5 ) formed from the given amount of oxygen and excess phosphorus. Now, determine the number of moles of P2O5 is produced from the given amounts of phosphorus and oxygen

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fespinoza019
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1
You know the right answer?
In Part A, you found the number of moles of product (1.80 mol P2O5 ) formed from the given amount of...
Questions in other subjects:


English, 24.10.2020 03:10

English, 24.10.2020 03:10






Mathematics, 24.10.2020 03:10


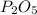 is formed by 4 moles of
is formed by 4 moles of 

 required =
required = 
 = 1.40 moles.
= 1.40 moles.

