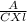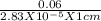| A solution containing 4.48 ppm KMnO4 exhibits
85.9 %T in a 1.00-cm cell at 520 nm. Cal...

Chemistry, 25.03.2020 16:11 Renabelle6350
| A solution containing 4.48 ppm KMnO4 exhibits
85.9 %T in a 1.00-cm cell at 520 nm. Calculate the
molar absorptivity of KMnO4 at this wavelength.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 02:30, micahwilkerson9495
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 04.04.2020 14:30






History, 04.04.2020 14:30


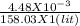 = 2.83 x 10⁻⁵ molar
= 2.83 x 10⁻⁵ molar