
Chemistry, 25.03.2020 06:43 letyourmemesbedreams
Consider the reaction: P(s) + 5/2 Cl2(g)PCl5(g) Write the equilibrium constant for this reaction in terms of the equilibrium constants, Ka and Kb, for reactions a and b below: a.) P(s) + 3/2 Cl2(g) PCl3(g) Ka b.) PCl3(g) + Cl2(g) PCl5(g) Kb

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 01:00, shartiarahoward
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Consider the reaction: P(s) + 5/2 Cl2(g)PCl5(g) Write the equilibrium constant for this reaction in...
Questions in other subjects:

History, 30.09.2020 04:01



Mathematics, 30.09.2020 04:01


English, 30.09.2020 04:01

Business, 30.09.2020 04:01



Mathematics, 30.09.2020 04:01


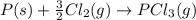
![K_a=\frac{[PCl_3]}{[Cl_2]^{\frac{3}{2}}}](/tpl/images/0562/7849/72fe4.png)
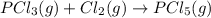
![K_b=\frac{[PCl_5]}{[Cl_2]\times [PCl_3]}](/tpl/images/0562/7849/4a569.png)

![K_c=\frac{[PCl_5]}{[Cl_2]^\frac{5}{2}}](/tpl/images/0562/7849/aecd1.png)
![K_c=K_a\times K_b=\frac{[PCl_3]}{[Cl_2]^{\frac{3}{2}}}\times \frac{[PCl_5]}{[Cl_2]\times [PCl_3]}](/tpl/images/0562/7849/835c8.png)


