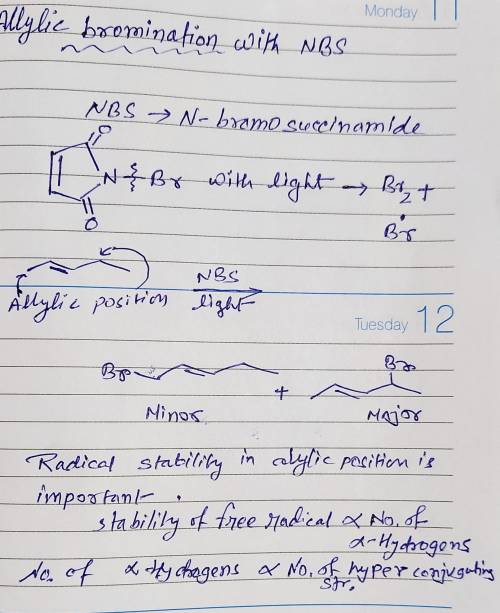
Chemistry, 25.03.2020 06:02 JocelynC24
Complete the mechanism and the products for the reaction of 2-pentene with N-bromosuccinimide (NBS) in light and carbon tetrachloride. Use curved arrows, bonds, atoms, and electrons to complete the mechanism and product(s). The first two steps are completed.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1


Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Complete the mechanism and the products for the reaction of 2-pentene with N-bromosuccinimide (NBS)...
Questions in other subjects:


Mathematics, 04.06.2021 02:30



Spanish, 04.06.2021 02:30


Advanced Placement (AP), 04.06.2021 02:30



Mathematics, 04.06.2021 02:30