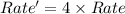Chemistry, 25.03.2020 07:01 mamas4539p79bw7
The reaction X + 2M → products has been found to have the rate law, rate = k[X] [M]2 . While holding the concentration of M constant, the concentration of X is increased from x to 4x. Predict by what factor the rate of reaction increases.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1


Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
The reaction X + 2M → products has been found to have the rate law, rate = k[X] [M]2 . While holding...
Questions in other subjects:







Social Studies, 31.10.2019 04:31

English, 31.10.2019 04:31

Biology, 31.10.2019 04:31

Biology, 31.10.2019 04:31

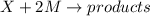
![Rate=k[X][M]^2](/tpl/images/0562/8462/02d02.png)
![Rate'=k[4X]^1[M]^2](/tpl/images/0562/8462/66381.png)
![Rate'=k[4]^1[X]^1[M]^2](/tpl/images/0562/8462/31177.png)