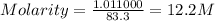Chemistry, 25.03.2020 05:31 wallsdeandre25521
Hydrochloric acid is usually purchased in concentrated form with a 37.0% HCl concentration by mass and a density of 1.20 g / mL. How much of the concentrated stock solution in milliliters should you use to make 2.5 L of 0.500 M HCl

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1


You know the right answer?
Hydrochloric acid is usually purchased in concentrated form with a 37.0% HCl concentration by mass a...
Questions in other subjects:




Mathematics, 05.04.2021 22:20


Chemistry, 05.04.2021 22:20




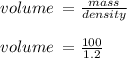
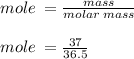
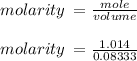
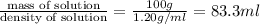
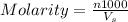
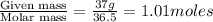
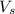 = volume of solution in ml = 83.3 ml
= volume of solution in ml = 83.3 ml