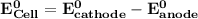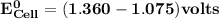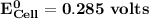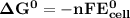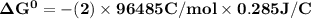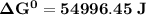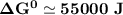Chemistry, 25.03.2020 05:26 kenzie3497
Determine ΔG° for a cell that utilizes the following reaction: Cl2(g) + 2Br–(aq) → 2Cl–(aq) + Br2(l) The standard reduction for the chlorine gas is 1.360 volts and the standard reduction for the bromine liquid is about 1.075 volts. Group of answer choices

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1


Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Determine ΔG° for a cell that utilizes the following reaction: Cl2(g) + 2Br–(aq) → 2Cl–(aq) + Br2(l)...
Questions in other subjects:

History, 20.10.2021 03:20

Health, 20.10.2021 03:20


Mathematics, 20.10.2021 03:20



Mathematics, 20.10.2021 03:20

Mathematics, 20.10.2021 03:20


Chemistry, 20.10.2021 03:20

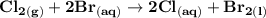
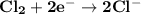
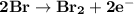
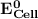 can be calculated as:
can be calculated as: